Student Guidance on applying for Ethical Approval (Link to Participation and Consent form Template that MUST be used)
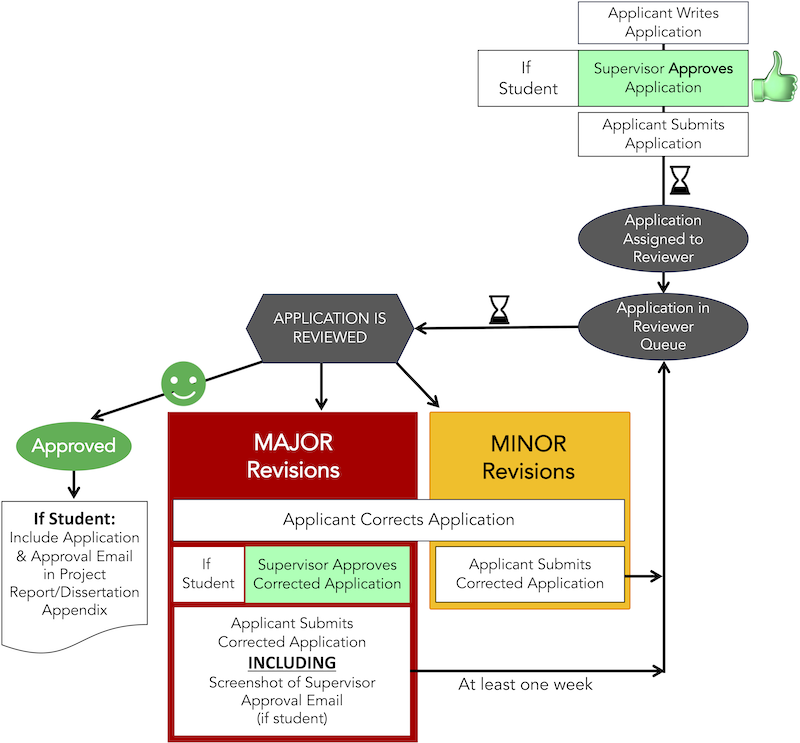
Ethical Application Approval Process
You can use the Ethics Approval System to for making your application.
The purpose of the Departmental and University Ethics Committees is to ensure that any investigations (regardless of subject domain) carried out by staff, or students that use human beings as participants are known to conform to the standards set by the professional bodies. These standards are aimed at ensuring that the rights, safety, and wellbeing of the participants is taken into account at all times.
Contact
The contact for applicants about information related to ethical approval of studies is cis-ethics@strath.ac.uk.
It is important to realise that any investigation that involves human beings as participants – even something as apparently harmless as a questionnaire or simple user study being undertaken as part of a project or your research – has to be granted ehtical approval. To gain this approval you need to provide a protocol describing how you plan to conduct your study via the Departmental Ethics Approval system at:https://local.cis.strath.ac.uk/wp/extras/ethics/The protocol will be expected to cover the following points:
- How participants will be recruited (i.e. how you will obtain volunteers).
- How participants’ consent will be obtained and demonstrated (this needs to be auditable).
- What participants will be told about the conduct of the research (i.e. they need to be briefed to be able to make an informed decision about whether to participate).
- What participants will be expected to do (in broad terms – see the point about content below). Please note that any participant must be able to freely withdraw from the study at any point (if they begin to feel stressed for example) and must not feel under any pressure or obligation to complete the study, answer any particular question, or undertake any particular task.
- How data will be stored (i.e. how the security of analogue and digital data will be maintained).
- How data will be processed (e.g. analysed, reported, visualised, integrated with other data, etc., all of which must adhere to the Data Protection Act and take participant confidentiality into consideration).
- How and when data will be disposed of (i.e. comprehensive and secure destruction).
These points cover most studies carried out within the department, but you should read the extract below from Appendix 2 of from the University’s Code of Practice on Investigations on Human Beings to make sure that there are not any other issues you should be considering. Note that for more substantial or unusual projects additional information may be required and closer reading of the full Code of Practice (available from the University Ethics Committee page) and discussions with the relevant departmental contact.
Extract from the University’s Code of Practice on Investigations on Human Beings
Annex 2: Guidance on Compiling Information sheets and Consent Forms:
A template for both the information sheet and consent form can be found on the website www.strath.ac.uk/ethics. All investigators are asked to take account of the following checklist. This checklist also provides guidance on the expected content of the information sheet for volunteers.1. INFORMED CONSENT
Check list for points that should be mentioned on all consent forms, which participants need to sign.1. Their participation is voluntary.
2. Their signature on the consent form indicates:
(a) that they are aware of what their participation involves, and of any potential risks;
(b) that all their questions concerning the investigation have been satisfactorily answered.
3. They can terminate their participation at any time without giving a reason and without any of their rights being affected (this is particularly important for students who might be concerned that it could be counted towards their success in their course).
4. They can also ask to have their data withdrawn from the investigation.
5. They are under no obligation to respond to all aspects of the investigation: for example, they can refrain from answering any survey question(s) about which they feel uncomfortable.
6. They understand that all information they give will be treated with the utmost confidentiality and their anonymity will be respected at all times.
7. Where relevant, they give their consent to the investigators to access specified records, e.g. medical notes.
8. Where relevant, they give permission for the investigator to maintain records of the investigation should a follow-up to the investigation be conducted in the future, or a further investigation be undertaken.
9. For investigations where it has been decided that ‘no fault compensation’ cover will be provided the following wording should be included in the consent form:
‘In agreeing to participate in this investigation you should be aware that you may be entitled to compensation for accidental bodily injury, including death or disease, arising out of the investigation without the need to prove fault. However, such compensation is subject to acceptance of the Conditions of Compensation, a copy of which is available on request.’
10. Where human biological samples are taken (e.g. blood samples or biopsy samples) then the following statement should be included in the consent form – ‘All human biological samples will be the property of the University of Strathclyde’. Where it is proposed to carry out DNA analysis of material in any samples then the following statement should be included in the consent form – ‘I consent to DNA in the samples being analysed’.
2. INFORMATION FOR PARTICIPANTS
The guiding principle is that all participants should be told as much as they might reasonably be expected to know in terms they will understand, about the purpose and procedures involved in the investigation, in order to be able to make an informed choice about whether or not they wish to participate. List of items which should be addressed on the Information Sheet for participants.1. Background and purpose of the investigation with possible benefits.
2. Investigator(s)’ name, affiliation and contact details.
3. Status/role of investigator(s) (e.g. staff, undergraduate/postgraduate student).
4. Funding body for the investigation (if applicable).
5. Nature of the participants sample: any screening procedures necessary; any inclusion/exclusion criteria; any special skills/attributes involved.
6. Confidentiality and anonymity of participant details. While confidentiality and anonymity must be guaranteed by the researchers, there are certain circumstances where information provided by the participant may have to be disclosed to others e.g. where someone is at risk of harm or has been harmed. This will be discussed with the participant at the time so that they are aware of this and can be involved (or not) in how best to pass this information on. The participant’s identity and any personal identifier information should not be disclosed.
7. The nature of the investigation and what is involved for participants.
8. Duration and location of the investigation and participant timetable.
9. Any potential risks or discomfort for participants, any burdens imposed, any specific preparatory requirements (e.g. special diet, exercise).
10. Any payment/reimbursement to be made.
11. Person to whom questions/concerns should be directed before, during or after the investigation. Plus the name of an independent person to whom any questions may be directed or further information may be sought from – this is normally the secretary to the Ethics Committee.
12. Adequate debriefing/feedback after participation.
13. Where participants are minors or otherwise unable to give their full consent, a named individual responsible for their care will be asked to provide assent or to consent on their behalf.
14. Ethical approval has been obtained/is being sought.
15. Refusal to participate or withdrawal from an investigation should not affect any other aspects of the way a person is treated (e.g. best medical care)
16. How the data from the investigation will be stored, how long it will be retained and, where appropriate, if it will be used in any other future investigation.
